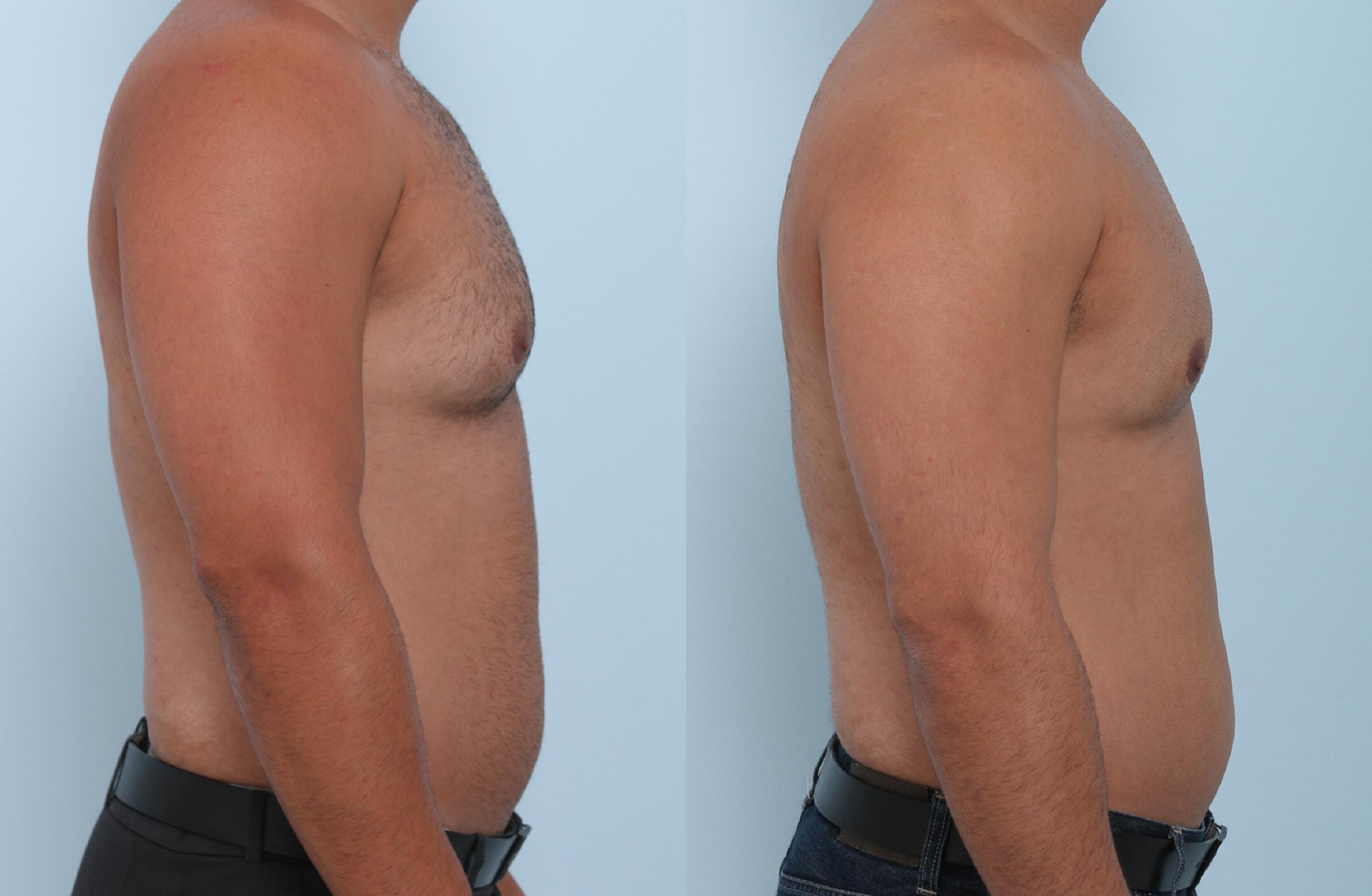
Body contouring Lewisville
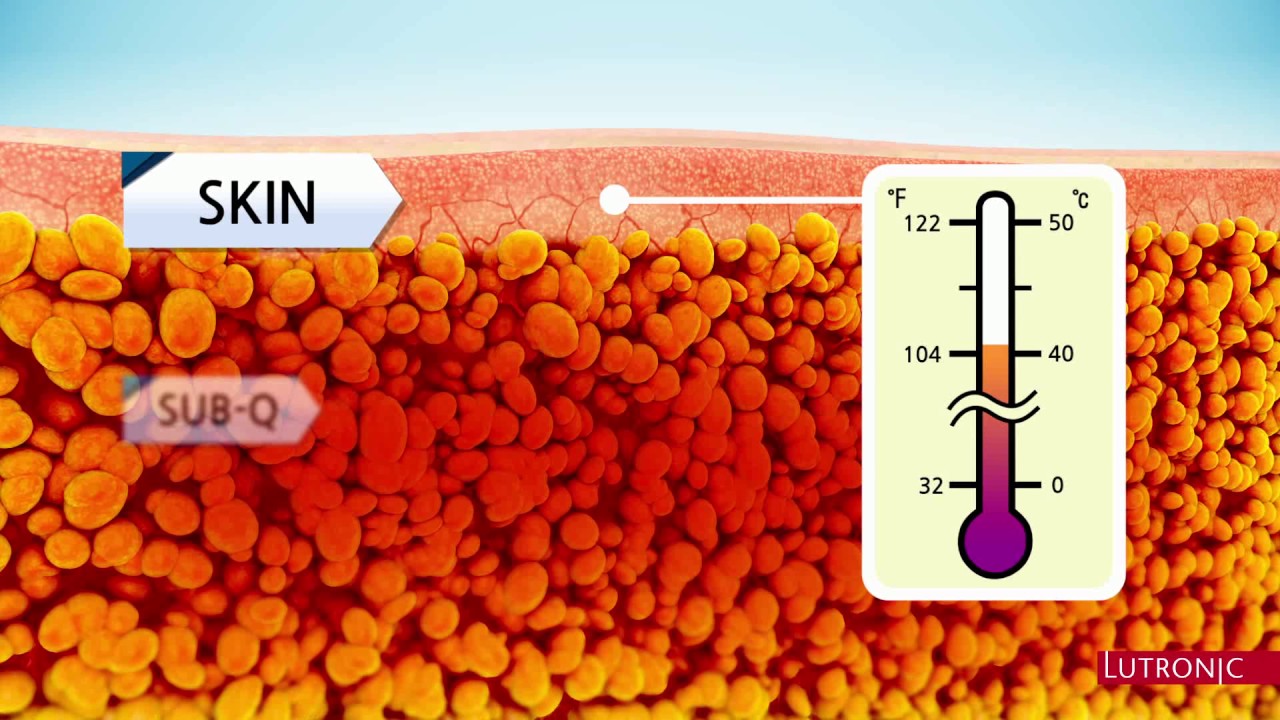
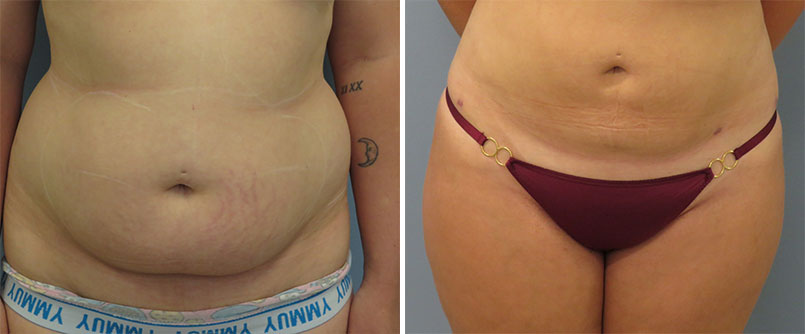
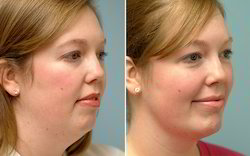
Dr. Jegasothy has seen positive results from using Kybella in these areas. Kybella was approved in August 2015, according to the FDA. I have also been using Kybella on other parts of my body than the neck, for very limited and very carefully selected patients. Dr. Jegasothy also pays close attention to certain areas that carry blood vessels and try to avoid those areas. I usually inject the love handles, the saddlebags, the area around the waist, and occasionally the fat that is superficially located on the anterior part of the abdomen. I am able to treat patients who are within 10 to 15 percent of their ideal weight. I do not have any results with patients who are heavier than that. They are less satisfied with my treatment results.